The Amgen emblem is displayed outdoors Amgen headquarters on Could 17, 2023 in Thousand Oaks, California.
Mario Tama | Getty Photos
Amgen is taking a new approach because it tries to face out in a crowded area of drugmakers racing to develop the following blockbuster weight loss drug.
The biotech firm is testing an injectable treatment that helps individuals shed some pounds in another way from the prevailing injections from Novo Nordisk and Eli Lilly, and different weight problems medicines in improvement. Amgen’s therapy, known as MariTide, additionally seems to assist sufferers preserve weight off after they cease taking it.
The drugmaker can be testing its drug to be taken as soon as a month and even much less regularly, which might provide extra comfort than the weekly medicines available on the market.
It is too early to say how aggressive Amgen shall be within the budding weight reduction drug area, which Novo Nordisk and Eli Lilly have to this point dominated.
Some analysts anticipate the market could possibly be price $100 billion by the top of the last decade, probably leaving room for brand new opponents to enter. Goldman Sachs additionally projects that between 10 million and 70 million Individuals shall be taking weight reduction medication by 2028.
The accessible knowledge on Amgen’s injectable drug is promising, nevertheless it’s from a small, early-stage clinical trial. The Thousand Oaks, California-based firm is also growing an oral medication and different therapies for weight problems, however has disclosed few particulars about them.
Buyers and well being specialists will possible get a greater thought of Amgen’s prospects later this 12 months: The drugmaker expects to launch preliminary knowledge from an ongoing mid-stage trial on MariTide, together with part one knowledge on its weight problems capsule.
It is also unclear whether or not Amgen’s therapies shall be cheaper than the prevailing weight reduction medication, which value round $1,000 per thirty days.
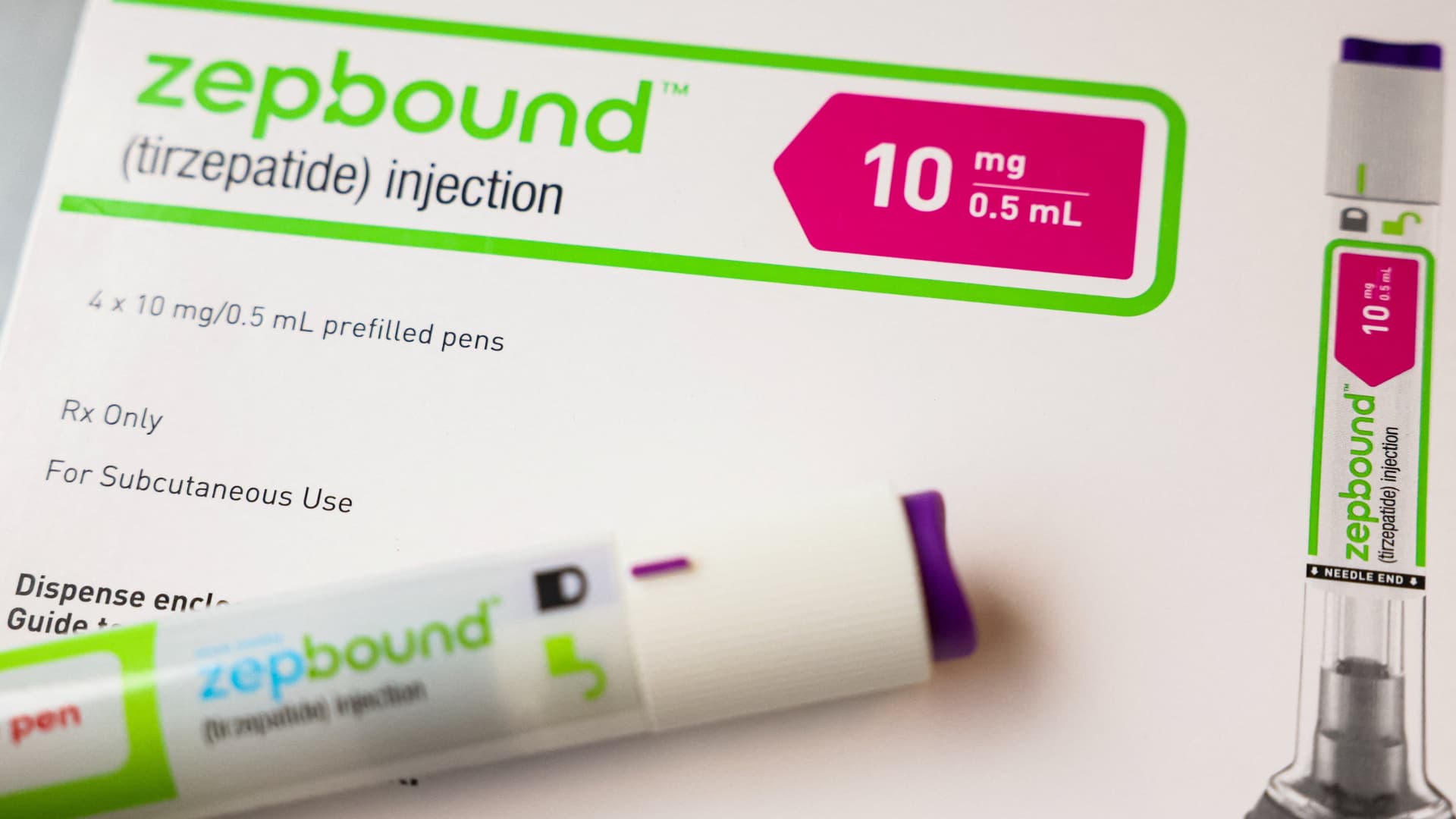
Wegovy from Novo Nordisk and Zepbound from Eli Lilly lead a brand new class of weight problems therapies that has drawn unrelenting affected person demand — and investor curiosity — regardless of their hefty worth tags and restricted insurance coverage protection.
Eli Lilly and Novo Nordisk have additionally struggled to supply sufficient provide of their therapies, which might give different corporations an opportunity to win market share.
How Amgen’s therapy is totally different
Amgen’s drug gives a new twist on weight reduction.
Very similar to Wegovy and Zepbound, one a part of Amgen’s therapy prompts a intestine hormone receptor known as GLP-1 to assist regulate an individual’s urge for food.
However whereas Zepbound prompts a second hormone receptor known as GIP, Amgen’s drug blocks it. Wegovy doesn’t goal GIP, which suppresses urge for food like GLP-1 however may enhance how the physique breaks down sugar and fats.
Amgen’s determination to tamp down reasonably than increase GIP exercise is predicated on genetics analysis suggesting that blocking the receptor is linked to decrease fats mass and physique weight, firm executives have mentioned.
Some permitted and experimental weight reduction medication
- Wegovy from Novo Nordisk: Permitted weekly injection that prompts GLP-1
- Zepbound from Eli Lilly: Permitted weekly injection that prompts GLP-1 and GIP
- Saxenda from Novo Nordisk: Permitted weekly injection that prompts GLP-1
- MariTide from Amgen: Experimental month-to-month injection that prompts GLP-1 and blocks GIP
- Danuglipron from Pfizer: Experimental once-daily capsule that prompts GLP-1
- VK2735 from Viking Therapeutics: Experimental weekly injection that prompts GLP-1 and GIP
- Pemvidutide from Altimmune: Experimental weekly injection that prompts GLP-1 and one other intestine hormone known as glucagon
- GSBR-1290 from Construction Therapeutics: Experimental weekly capsule that prompts GLP-1
- Survodutide from Zealand Pharma, Boehringer Ingelheim: Experimental weekly injection that prompts GLP-1 and glucagon
That seems to contradict how Zepbound works. Eli Lilly’s method has confirmed profitable: The therapy helped sufferers with weight problems lose as much as 22.5% of their weight after 72 weeks in a late-stage trial.
However Amgen’s MartiTide additionally was efficient in a small, early-stage examine.
Sufferers given the very best dose of Amgen’s drug — 420 milligrams — each month misplaced 14.5% of their physique weight on common in simply 12 weeks, in accordance with data from the phase one trial revealed final month within the journal Nature Metabolism.
There is a broader debate amongst researchers about why each approaches – blocking and activating GIP – are efficient at selling weight reduction.
One theory is that repeatedly activating the GIP receptor, as Zepbound does, in the end causes the physique to “self-regulate” itself and ensure there is not an excessive amount of GIP exercise, mentioned Dr. Caroline Apovian, a director of the Heart for Weight Administration and Wellness at Brigham and Ladies’s Hospital.
That decreases GIP exercise general, which is believed to primarily mimic what Amgen’s drug achieves when it blocks the GIP receptor. However Apovian cautioned that “none of this is proven” and extra knowledge is required.
The drug might lead to longer-lasting weight reduction
Amgen’s therapy could also be higher at serving to individuals preserve weight reduction than opponents, although sufferers take it much less regularly, early-stage trial knowledge suggests.
Amgen’s examine enrolled 110 sufferers with weight problems however not diabetes. Sufferers in a single group had been randomly assigned to obtain a single dose of the drug and had been adopted for 150 days, whereas a second group was given a dose each 4 weeks for 3 months.
An weight problems affected person takes a injection of weight reduction treatment.
Joe Buglewicz | The Washington Submit | Getty Photos
Sufferers who obtained a single shot of the very best dose of MariTide misplaced as much as 8.2% of their physique weight after 92 days. That means a single injection of the drug has a protracted weight reduction impact, in accordance with the examine authors.
Within the group that obtained a number of doses of the drug, sufferers appeared to take care of their most weight reduction till round two months after their final dose. Their physique weight began to slowly return after that. Nonetheless, their weight was as a lot as 11.2% decrease 5 months after they obtained the final dose.
“We think meaningful weight loss is already 5%. If you take Amgen’s drug, lose 14.5%, stop the drug and still have 11.2% weight loss after a few months, that’s significant,” mentioned Dr. Holly Lofton, director of the Weight Administration Program at NYU Langone Well being and an weight problems medication doctor. However she identified the necessity to examine the therapy in a bigger group of individuals.
The sustained weight reduction in Amgen’s examine seems to distinction with outcomes seen in medical trials on Zepbound and Wegovy. Sufferers in these research noticed their weight rebound sooner after stopping the injections.
As soon as a month and even much less frequent dosing
The frequency of Amgen’s drug additionally units it aside. These on Wegovy or Zepbound need to take doses weekly, in contrast with the once-monthly MariTide.
Amgen’s trial used month-to-month dosing partly as a result of sufferers noticed sustained weight reduction whether or not they had a single injection or a number of pictures of the corporate’s drug, in accordance with the examine authors.
Amgen’s therapy can also keep within the physique for for much longer than present therapies like Wegovy and Zepbound as a result of it features a monoclonal antibody, the authors added.
An injection pen of Zepbound, Eli Lilly’s weight reduction drug, is displayed in New York Metropolis, U.S., December 11, 2023.
Brendan McDermid | Reuters
Amgen’s MariTide “has that advantage where it’s just going to last a lot longer. Even if you give a high dose, you’re still going to have drug exposure in the body for a month or two months, so that clearly shows you don’t need to take it every week,” William Blair & Firm analyst Matt Phipps instructed CNBC.
Phipps mentioned individuals usually do not wish to get injections usually, so some sufferers might favor a month-to-month shot like Amgen’s MariTide for a illness that may possible require power therapy.
However he famous {that a} affected person’s alternative may depend upon whether or not the extent of weight reduction and unwanted effects of Amgen’s drug find yourself being on par with these of the prevailing weekly injections.
Amgen’s ongoing part two trial is exploring whether or not sufferers can take its drug even much less regularly than as soon as a month.
Section two trial will deliver extra readability
Amgen’s longer-term part two examine on almost 600 sufferers will present extra readability on how aggressive MariTide shall be towards Wegovy and Zepbound. The corporate is exploring which dose power and schedule is greatest for sufferers. It expects to launch preliminary trial outcomes later this 12 months.
Some analysts have mentioned the part two trial might assist handle a number of questions, together with how effectively sufferers tolerate the therapy at totally different dose regimens.
The 52-week examine is testing 11 totally different affected person teams at a wide range of dosing ranges and regimens. That features beginning some sufferers at a decrease dose of a drug and progressively rising it till they attain the next goal dose.
That dose escalation might assist scale back unwanted effects that some sufferers skilled after taking their first dose of MariTide within the part one trial, in accordance with Phipps.
In that trial, the protection and unwanted effects of Amgen’s drug had been much like different GLP-1 drugs. Nausea and vomiting had been essentially the most generally reported unwanted effects, and usually lasted for about 72 hours.
4 out of eight sufferers in a gaggle receiving the very best dose of the therapy withdrew earlier than getting a second shot on account of gentle gastrointestinal points, in accordance with the examine. However no different sufferers stopped taking the drug on account of opposed occasions throughout any of the totally different dosing teams, Amgen Chief Medical Officer Paul Burton mentioned throughout a convention earlier this month.
“It’s a little early to jump to the conclusion that the drug won’t be tolerated by patients based on this phase one data,” William Blair & Firm’s Phipps mentioned.
One other a part of Amgen’s part two trial may even look at weight reduction past 52 weeks, which is able to present a clearer image of how lengthy the drug is efficient.