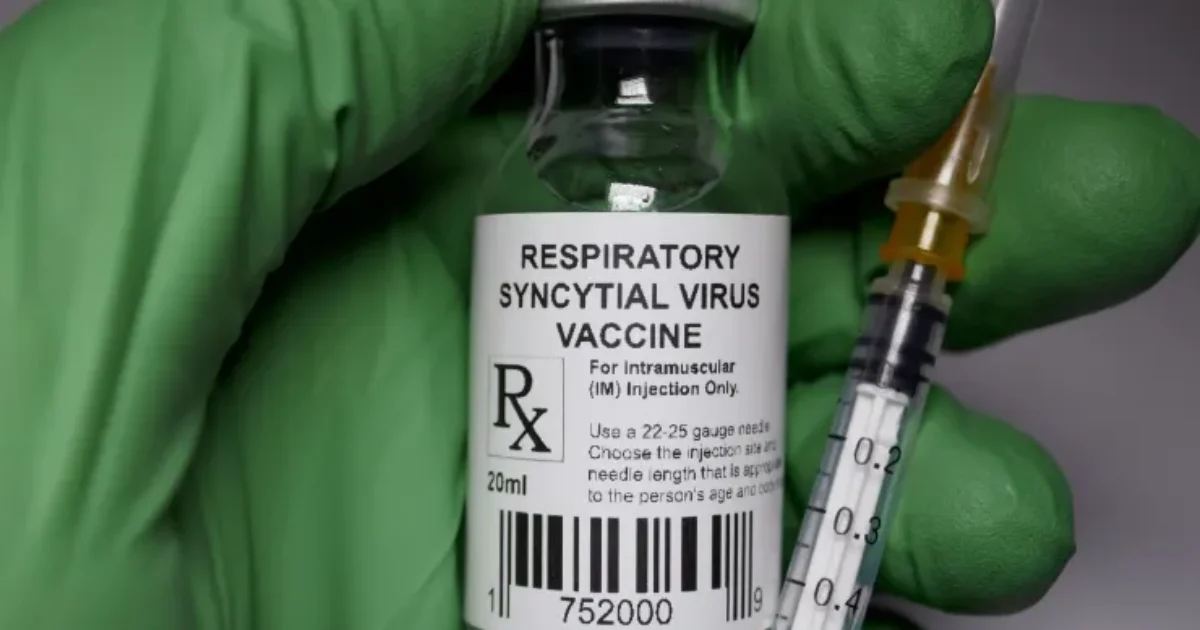
The Food and Drug Administration (FDA) has recently approved an expansion of a respiratory syncytial virus (RSV) vaccine for use in at-risk adults over the age of 50.
Manufactured by British multinational pharmaceutical and biotechnology company GSK, this vaccine is the first to be approved for adults aged 50–59, targeting a virus known for causing severe respiratory issues, particularly in older adults and those with pre-existing health conditions.
RSV is a common respiratory virus that primarily infects the lungs, nose, throat, and breathing passages. It typically causes mild cold-like symptoms such as a runny nose, fever, cough, and wheezing.
“Today’s approval reflects the importance of broadening the benefits of RSV immunization to adults aged 50-59 who are at increased risk,” said Tony Wood, GSK’s chief scientific officer. “For those with underlying medical conditions, RSV can have serious consequences, so we are proud to be the first to help protect them from RSV-LRTD.”
Professor Ann R. Falsey, University of Rochester School of Medicine, said, “I am thrilled that GSK’s RSV vaccine is now approved for adults aged 50-59 at increased risk of RSV-LRTD. When it comes to the risks associated with RSV, age is just a number, an important number, but not the only factor to consider. Many adults in this age group have underlying health conditions that place them at increased risk for serious illness with RSV infection compared with those without these conditions. Now there is a vaccine approved that can help protect them.”
Now, GSK awaits a recommendation from the Centers for Disease Control and Prevention’s (CDC) immunization advisory committee for those in this age group, according to The Hill.
The CDC panel will also have to consider the potential need for RSV booster shots.
Other major pharmaceutical companies like Moderna and Pfizer also produce RSV vaccines approved for adults older than 60 years. The FDA approved Moderna’s RSV vaccine late last month, making it the third vaccine greenlighted to fight against the disease, following GSK’s Arexvy and Pfizer’s mRESVIA.
In March, The Gateway Pundit reported that the Food and Drug Administration’s advisory committee voted in favor of approving the world’s first respiratory syncytial virus (RSV) vaccine for adults 60 and up from Pfizer.
Scientists recommend that older people who received Pfizer’s RSV vaccine should be monitored for Guillain-Barre syndrome.
Specialists who voted against the vaccine based on its safety profile were concerned that it might increase the risk of Guillain-Barré syndrome (GBS), a rare neurological disease that can kill nerve cells and lead to muscle weakness or paralysis.