The biotech sector has embraced the microbiome in recent times, a inexperienced discipline market powered by low-cost genome sequencing and enterprise {dollars}, promising bespoke therapies for every thing from intestine troubles to pores and skin issues. However a report in Science alleges that these corporations lack scientific rigor and significant regulation, providing little greater than guesswork on a fancy and understudied space of human well being. The startups in query provide a nuanced response to this criticism that emphasizes their efforts to attain legitimacy in an space they admit has the potential for snake oil.
The microbiome is a common time period for the distinctive mixture of micro organism and different microorganisms that every of us has in and on our our bodies. Your pores and skin, intestine, mouth, genitals, and any variety of different components have a wealthy number of resident life, from these regarded as longstanding and useful to newer, rarer, and even “invasive” ones.
What many corporations have proposed is that by profiling one’s microbiome, many associated well being issues or advantages could be recognized, handled, or promoted. This profiling is achieved by mass genomic evaluation of a swab, stool pattern, or another organic supply, a course of that (the businesses say) quantifies the nice, unhealthy, and ugly life endemic to your physique.
However six students, citing a wide range of researchers, clinicians, sufferers, and different specialists they interviewed, warn towards what they see as a largely unregulated business rife with snake oil. (This isn’t a peer-reviewed analysis paper, however nonetheless depends on unique analysis.)
“These companies claim they can determine whether a customer’s microbiome is healthy or in ‘dysbiosis’ — out of balance — and suggest that if so, it could be the reason for one or more health problems,” the group writes. “Some of these companies may knowingly mislead consumers, while most appear to engage in questionable practices that are permitted by gaps in the current regulatory framework.”
The issue just isn’t with the concept of microbiome profiling in itself. As microbiologists and researchers on microbiome points, they’re properly conscious of the potential for, say, an irregular intestine microbiome to contribute to any variety of well being issues. The scientists — and entrepreneurs — that I’ve talked to all agree that this can be a potentially transformative area of research and medication that has solely within the final decade turn out to be sensible to check at scale.
It is because the worth of analyzing genetic supplies has dropped via the ground, whereas the benefit of dealing with the massive and complex dataset that outcomes from such testing has risen equally quick. The outcome is a humiliation of information, through which may very properly be hidden a therapy path for a lot of an individual’s well being points.
The place the authors take subject is with the presentation of microbiome profiling and therapy as established science and the dearth of regulation that stops them from making these claims.
“Many of their marketing claims imply, and may lead consumers to believe, that the results are grounded in scientific accuracy and are medically relevant when that has not been substantiated,” they write.
Though many corporations tout research, these are sometimes internally carried out and, as a result of they’re based mostly on proprietary information, tough if not unimaginable to copy.
Viome, for example, which raised $86 million last year, cites a study supporting its declare that its service may help folks with their irritable bowel syndrome, melancholy, and diabetes. I lack the experience to guage the examine, but it surely and others prefer it are all by Viome workers, on Viome information, utilizing Viome strategies. Although it appeared within the American Journal of Life-style Drugs, it has not (like many papers, to make certain) been cited besides by subsequent Viome research. (I requested Viome for touch upon the arguments of the Science piece, however didn’t hear again as I did from the others.)
It’s as much as you and your physician to determine if that is adequate scientific backing for a nutrition-based strategy to treating your diabetes. However the Science piece’s authors assert that every one these research are carried out on shaky grounds, as a result of there may be merely no accepted, foundational understanding of microbiomes and their results on any bodily processes in any way.
“The companies need consistency in processes and methods to ensure consistency across labs, but they also need reference standards with which to compare their results and make sure if they say a consumer’s results are within a ‘normal’ range, they are in fact consistent with what would be a ‘normal’ range,” co-author Dianne Hoffman, of the College of Maryland, instructed TechCrunch. “That has not as yet been established.”
“There is no consensus about what constitutes a healthy human microbiome composition in any population or subpopulation,” the report states.
But microbiome corporations inform customers not simply what’s or isn’t wholesome, but additionally what they will do — and purchase — to enhance it. Almost half the businesses surveyed by the authors promote the dietary supplements they advocate, and naturally advocate you do a number of follow-up exams on their platform to trace their results.
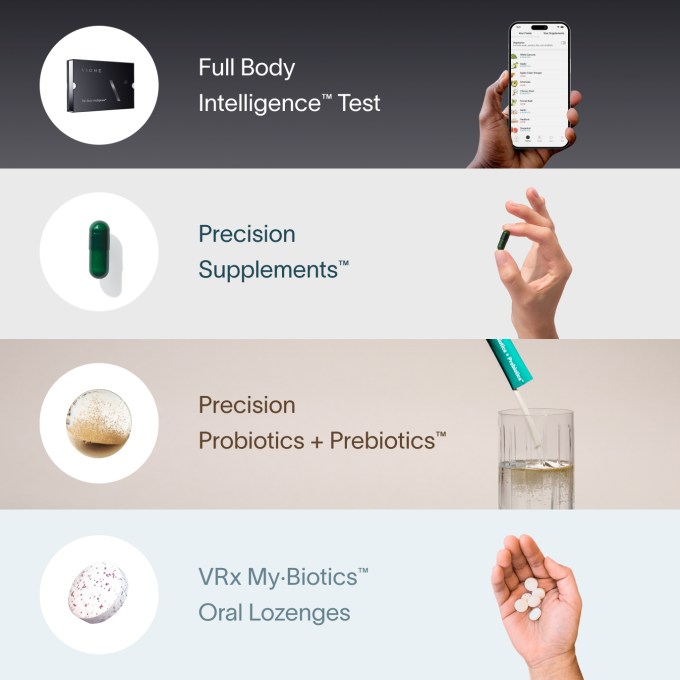
Viome’s front-page testing-through-supplements pitch. Picture Credit: Viome
Even when there have been that foundational data, the authors level out that the testing processes utilized by microbiome corporations “have been shown to lack analytical validity, resulting in inconsistent test results from the same sample across different laboratories as well as within the same laboratory.” This isn’t a blanket assertion that they’re inaccurate or that there’s nothing corporations can do to mitigate the exams’ shortcomings, however that there is no such thing as a normal and no requirement to take action.
The FDA, they observe, doesn’t regulate these corporations, since every is cautious concerning the extent and wording of their claims and don’t say that, for instance, their dietary supplements are a full-on therapy for a illness. As an alternative, they could be described as bettering outcomes, or advancing holistic well-being, or the rest you would possibly discover on the packaging of a complement.
The Facilities for Medicare & Medicaid Providers (CMS) nominally regulate them, as a result of their labs have to be run inside sure authorized limits. However because the authors level out, microbiome testing falls between the cracks as a result of it’s not supposed to discover a specific pathogen or degree. They will get CMS certification with out displaying proof that their exams are correct. Once more, that is no assure that they’re inaccurate, however one should admit it’s troubling to listen to that so little is outwardly required of them.
However one may additionally ask, what’s unsuitable with somebody getting extra data and taking a complement that, like so many, is at worst ineffective somewhat than actively dangerous? The report cites a danger of “self-misdiagnosis, delay in seeking medical treatment, and substituting nonmedical supplements for prescription medications.”
“There are patients with chronic gut illnesses, including children whose parents are searching for cures for their children, who are following nutritional or supplement recommendations that are actually harmful to them. One physician we heard from shared that he has heard of patients doing a DIY fecal microbiota transplant on the basis of the results from a DTC microbiome based test,” co-author Diane Hoffman instructed TechCrunch.
Startups name criticism truthful for some, however not for all
The report is unsparing in its criticism of this nook of the business, but it surely paints with a broad brush and by necessity doesn’t delve into particular instances. I requested the leaders of three corporations that present microbiome testing for numerous causes what they consider the assertions made within the report above.
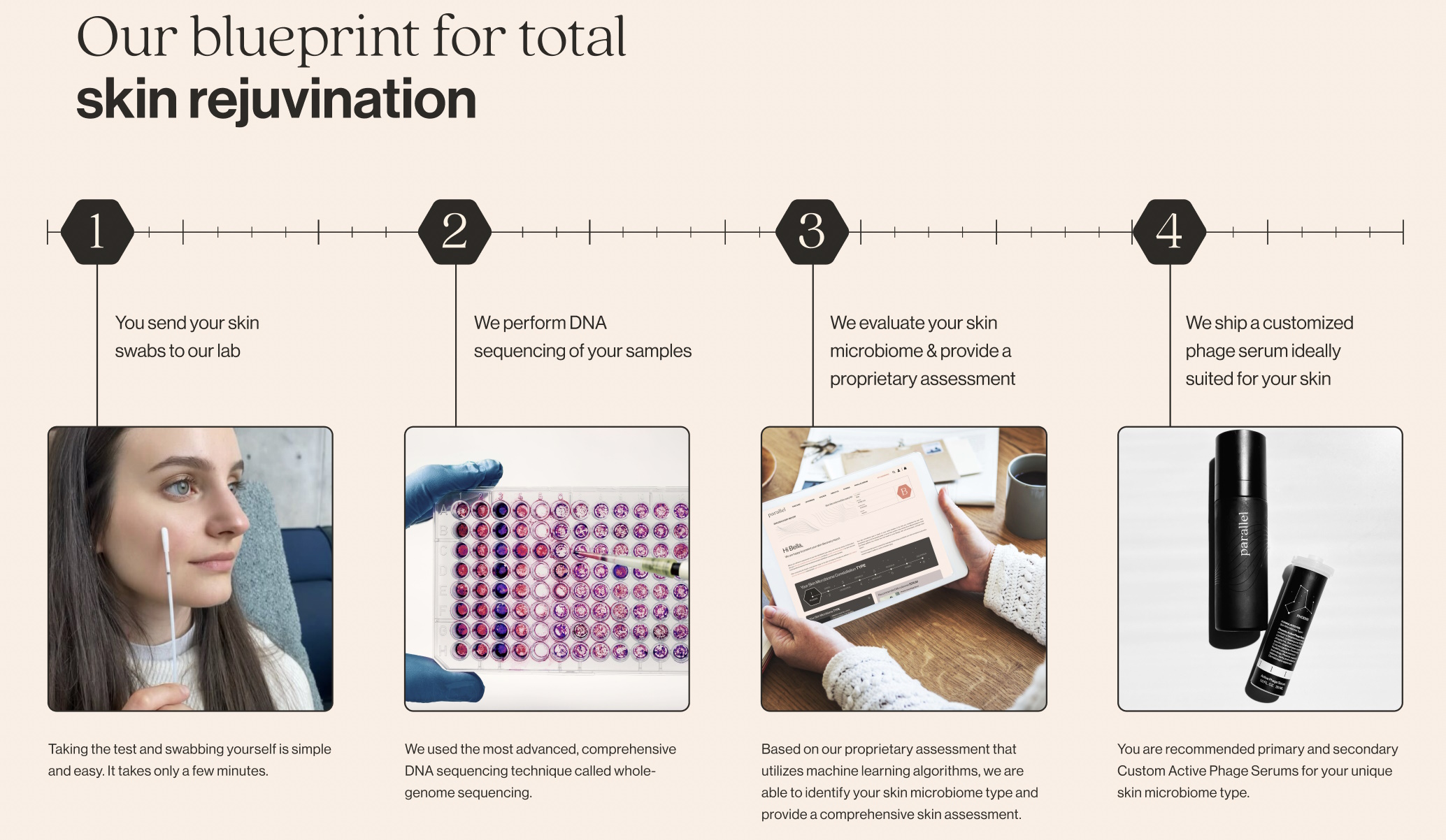
Natalise Kalea Robinson, co-founder and CEO of Parallel Health, which provides phage-based treatments catering to a customer’s skin microbiome, really agreed with lots of the factors, making it clear that it’s as much as the corporate to do higher.
“Concerns around analytical validity are warranted,” she stated. “This challenge is not only relevant to DTC microbiome companies but to all next-generation diagnostics companies at large. When you have technology advancing at the speed that it is, especially spurred by AI, it is hard for regulators to keep up. There is a very real and important hunger among consumers, and within at least some part of the microbiome industry, to finally develop a clinically validated human microbiome test. That we do not have one already is not a failure of regulation, but a lack of ambition in the microbiome industry.”

Picture Credit: Parallel Well being
The problem, she stated, is that the quantity of information wanted to provide a clinically validated, FDA-approved microbiome check means it may well’t be collected with out a firm like hers doing in order a enterprise. She agreed that there’s little consensus on what constitutes a “healthy” microbiome and that, because of this, that they had to make use of all of their preliminary funding to construct a broad, numerous dataset that may function one for his or her functions. (That is nonetheless proprietary, inside information, a fear from the report.)
Cheryl Sew Hoy, founder and CEO of Tiny Health, specializing in pediatric microbiome information, was fast to level out that whereas there could also be no scientific consensus on grownup intestine well being, there definitely is one for infants.

Picture Credit: Tiny Well being
“Because there are so few microbes in the early days — and the only function of the infant gut in the first 6 months is universally to digest milk and not a diversity of food — it’s much easier from a scientific perspective to characterize what is healthy or unhealthy in an infant’s gut, which is what Tiny Health specializes in,” she defined. “For that reason, the infant gut in the first 1,000 days is also much more malleable, only stabilizing after 3 years of age. Therefore, there’s much more scientific validation of what constitutes a healthy development of the infant’s immune system, which is intrinsically tied to the infant’s gut development.”
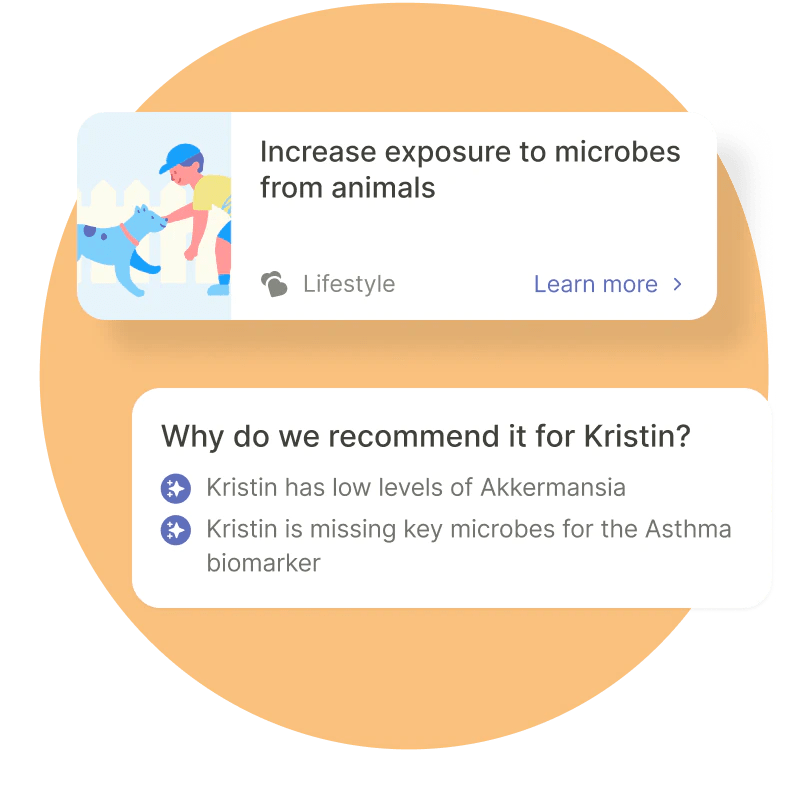
She stated that the corporate doesn’t diagnose, treatment or deal with any circumstances or present medical recommendation. This can be strictly true, but it surely have to be stated that it does provide to “prevent or manage microbiome-related conditions like colic, eczema, food allergies, asthma, constipation and more,” admittedly with the assist of a health care provider, however some suggestions, like publicity to pets, are made in-app. So though they’re doing the correct factor, they’re additionally flying pretty near the solar in that sense.
That stated, Tiny Well being can also be laying the muse for turning into an FDA-approved diagnostic and therapy platform, working a pair of scientific research monitoring how their stories and proposals have an effect on a baby’s first 1,000 days. Sew Hoy additionally stated that they’re bettering consequence monitoring with pressure identification, so you possibly can inform if a specific probiotic is working (that business, she famous, is “presently the wild wild west and never all corporations/merchandise are created equal).
Daye is a microbiome startup focusing on women’s reproductive and sexual health, and founder Valentina Milanova was fast to set her firm aside from others (not simply because it additionally has to adjust to U.Okay. well being laws).
“In the US D2C landscape, the vaginal microbiome is traditionally tested with gene sequencing technologies, which have limitations, when it comes to the standardization and validation of the method. These technologies also identify a number of microorganisms that are largely under-researched and with unknown clinical utility,” she identified. Daye, then again, makes use of a PCR check that identifies solely a subset of identified, clinically important microorganisms. PCR testing additionally has a extra strong validation and high quality management system.
Milanova didn’t disagree that some microbiomes are under-documented, however stated the vaginal microbiome just isn’t certainly one of them. “There is a scientific consensus on what constitutes a healthy vaginal microbiome — one that is dominated by lactobacilli bacteria. These produce lactic acid and other antimicrobial substances that maintain a protective vaginal pH and inhibit opportunistic bacteria from growing and causing infection,” she wrote.
“Daye’s test enables us to measure the relative abundance of good bacteria, as well as that of opportunistic microorganisms, that cause BV and yeast infections when found in large amounts,” she continued, however this is just one step in a clinician-involved course of that should establish the particular pathogen earlier than recommending a therapy. Within the U.Okay., this may be accomplished inside Daye’s platform, however within the U.S., they work with a community of clinicians.
She additionally shared Kalea Robinson’s perspective that enterprise funding is “an imperfect mechanism for funding R&D and innovation … venture capital investors operate on a short timeline and venture-backed startups need to provide commercial results in timelines, which are often unreasonable for medical innovation.” They use grants and common earnings as properly to assist this necessary work.
So far as regulation, all three girls stated they welcome it, because it ought to assist guarantee constant and useful outcomes.
“We hope that the FDA will recognize that microbiome tests can help improve health outcomes if done right,” Kalea Robinson stated. She expects its authority to increase and embody their work eventually, and to that finish “we are doing the hard work now” to arrange for that.
For people who aren’t, the sense is that regulation is required to curtail this nascent and disorganized business’s equal of invasive and opportunistic microorganisms — even the well-funded ones.















