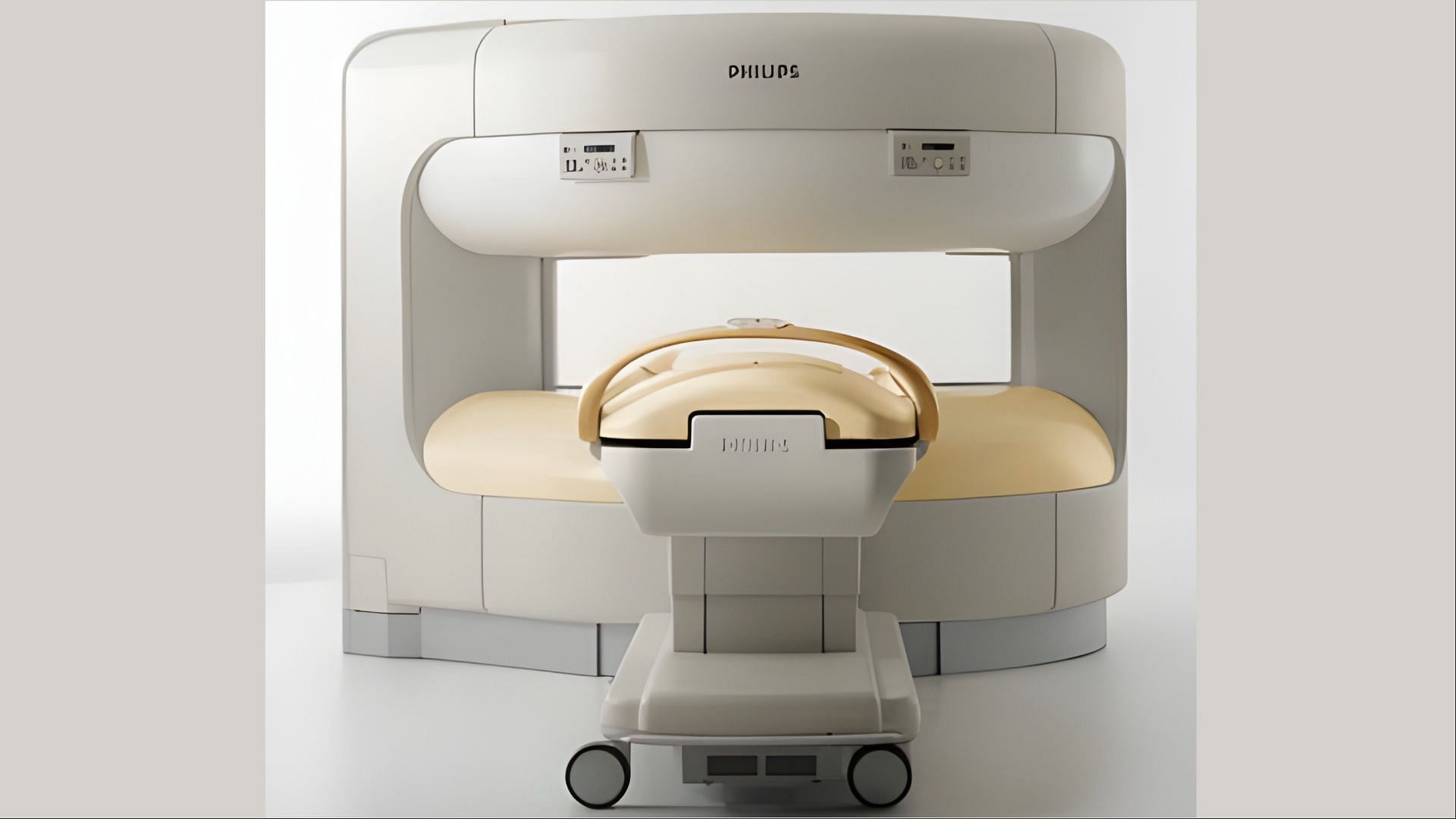
Over 150 Panorama 1.0T HFO MRI Scanners are being recalled by Philips North America LLC over explosion dangers. Feared to be attributable to extreme helium fuel buildup, the explosions will be life-threatening to each the affected person and care suppliers.
The voluntary recall issued on December 20 solely applies to the Panorama 1.0T HFO MRI Scanners that are primarily based on magnetic resonance (MR) programs and are broadly used at medical facilities and labs to scan the physique of the sufferers. The scanned outcomes are offered within the type of detailed digital pictures which can be utilized by the well being care suppliers for prognosis.
As per the institution, the affected MRI scanners might typically undergo a ‘quench process’ that’s identified to generate large quantities of helium. Whereas this fuel often evaporates and will get vented out of the constructing by a venting system, it might typically fail to take action if there is a blockage within the venting system.


It’s to be famous that the magnet geared up within the Philips Panorama 1.0T HFO scanners might undergo unintended quench procedures by itself or when the Magnet Emergency STOP button is pressed by the operator. In case the helium fails to vent by the system, it might result in extreme strain build-up and compromise the structural integrity of the system, thus posing dangers of an explosion.
All you’ll want to know in regards to the Philips Panorama 1.0T HFO MRI Scanner recall
Not less than 150 Panorama 1.0T HFO MRI Scanners produced by Philips are a part of a ‘Class 1’ recall initiated this Wednesday. Feared to pose dangers of an explosion, the affected medical gadgets might trigger each the sufferers and the caregivers to expertise critical to life-threatening accidents and even dying.
The institution has already knowledgeable the USA Meals and Drug Administration (FDA) about at the very least one explosion incident linked to the Panorama 1.0T HFO MRI Scanner. Whereas the explosion is reported to have resulted in some property and system damages, there have been no reviews of an harm or casualty. It’s to be famous that the involved scanners have been in use for over 22 years and this was the primary reported explosion.
The Panorama 1.0T HFO MRI Scanners affected by the recall got here with the product code ‘LNH‘ and have been obtainable underneath two mannequin numbers – 781250 and 781350. Distributed throughout the USA between January 1, 2001, and October 1, 2016, the recalled MRI Scanners have been formally discontinued in 2014.



Contemplating the graveness of the issue, Philips North America LLC has already despatched an ‘URGENT Medical System Correction‘ discover to all affected prospects. The discover urges all healthcare institutes utilizing the Panorama 1.0T HFO MRI Scanners to discontinue the utilization.
Well being-care suppliers are additionally urged to not provoke guide quench procedures except it is an emergency. The institution shall be sending a area service engineer to all United States customers. The engineer is not going to solely examine the affected scanners however can even carry out all corrective repairs and/or replacements if required.
People with queries in regards to the recall or the treatment can attain out to the Philips Buyer Care Options Heart at 1-800-722-9377.














