Pgiam/iStock through Getty Photographs

Verona Pharma plc (NASDAQ:VRNA) develops medicines for respiratory illnesses. Its lead (and solely) asset is ensifentrine, an inhaled and twin inhibitor of the phosphodiesterase (“PDE”) 3 and PDE4 enzymes. Ensifentrine is being developed in three formulations, as a nebulizer, a dry powder inhaler or DPI and pressurized metered-dose inhaler or MDI. Ensifentrine works as a bronchodilator and an anti-inflammatory agent in a single compound.
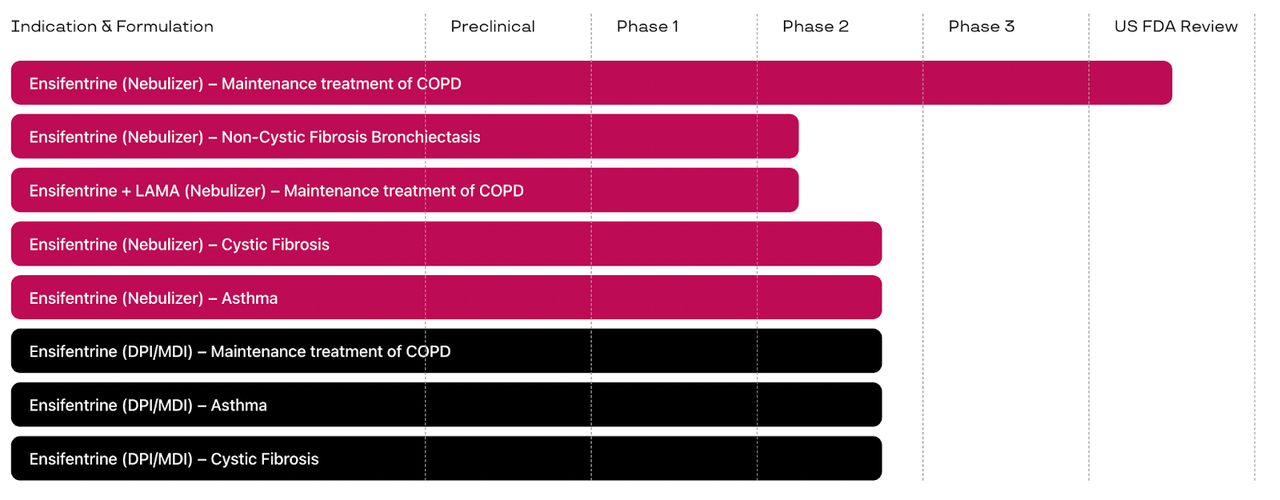
The pipeline is as follows:
Lead program is Ensifentrine as a nebulizer underneath FDA assessment after a profitable section 3 trial as upkeep therapy of COPD. PDUFA is on June 26. In the identical nebulizer formulation, it’s in 4 different section 2 trials in Non-Cystic Fibrosis Bronchiectasis, together with glycopyrrolate, a LAMA (Lengthy-Appearing Muscarinic Antagonist) in Upkeep therapy of COPD, in Cystic Fibrosis and in Bronchial asthma. Its DPI/MDI formulation is in 3 section 2 trials focusing on Upkeep therapy of COPD, Cystic Fibrosis and Bronchial asthma. A few of these section 2 trials appear to have not but begun, see their November press release. As in addition they point out of their earnings name:
…within the very close to time period, you need to anticipate R&D bills to be pretty restricted as a result of we’re not operating any scientific trials.
Three varieties of mechanisms of motion are presently in use – LAMA: Lengthy performing muscarinic agent, LABA: Lengthy performing beta-adrenoceptor agonist, ICS: Inhaled corticosteroids. The COPD house is very differentiated, and there are dozens of accredited medicine, nonetheless all of them use one or multiple of those mechanisms. Ensifentrine has a distinct MoA.
In 2022, the corporate posted optimistic section 3 knowledge from the ENHANCE-2 trial, which noticed a 42% discount in exacerbation charge over 24 weeks with ensifentrine versus placebo. It additionally met the first endpoint and key secondary endpoints with excessive p-value.
Right here’s the whole ENHANCE data:
Totals of 760 (ENHANCE-1) and 789 (ENHANCE-2) sufferers have been randomized and handled, with 69% and 55% receiving concomitant long-acting muscarinic antagonists or long-acting β2-agonists, respectively. Submit-bronchodilator FEV1 share predicted values have been 52% and 51% of predicted regular. Ensifentrine therapy considerably improved common FEV1 space underneath the curve at 0–12 hours versus placebo (ENHANCE-1, 87 ml [95% confidence interval, 55, 119]; ENHANCE-2, 94 ml [65, 124]; each P < 0.001). Ensifentrine therapy considerably improved signs (Evaluating Respiratory Signs) and high quality of life (St. George’s Respiratory Questionnaire) versus placebo at Week 24 in ENHANCE-1 however not in ENHANCE-2. Ensifentrine therapy decreased the speed of reasonable or extreme exacerbations versus placebo over 24 weeks (ENHANCE-1, charge ratio, 0.64 [0.40, 1.00]; P = 0.050; ENHANCE-2, charge ratio, 0.57 [0.38, 0.87]; P = 0.009) and elevated time to first exacerbation (ENHANCE-1, hazard ratio, 0.62 [0.39, 0.97]; P = 0.038; ENHANCE-2, hazard ratio, 0.58 [0.38, 0.87]; P = 0.009). Opposed occasion charges have been just like these for placebo.
The QoL and ERS lack of enchancment in ENHANCE-2 has been attributed to an unnatural placebo response. This sounds cheap as a result of ENHANCE-1 did meet these endpoints and there was not a lot distinction between ENHANCE-1 and ENHANCE-2 in addition to the truth that ENHANCE-1 consists of an extra security evaluation at week 48.
The present therapy panorama for COPD consists of the next drug courses:
LAMA: Lengthy-Appearing Muscarinic Antagonists. These are bronchodilator drugs that work by blocking the motion of acetylcholine, a neurotransmitter, within the airways. This results in the comfort of the muscle mass across the airways, leading to bronchodilation. Widespread LAMA drugs embrace tiotropium, aclidinium, and umeclidinium.
LABA: Lengthy-Appearing Beta-Agonists. LABAs are one other class of bronchodilators that work by stimulating beta receptors within the airway easy muscle mass. This stimulation leads to the comfort of the muscle mass and dilation of the airways. Examples of LABA drugs embrace salmeterol and formoterol.
ICS: Inhaled Corticosteroids. Inhaled corticosteroids are anti-inflammatory drugs that assist scale back irritation within the airways. They’re typically used to handle bronchial asthma and will also be a part of the therapy for COPD, notably together with LABAs. Examples of ICS drugs embrace fluticasone, budesonide, and beclomethasone.
COPD causes progressive obstruction of airflow, irritation and mucus manufacturing, affecting the standard of life via exacerbations and different signs. These 3 courses of medicines have been in existence for over 40 years, and no new medicine with a novel mechanism of motion has been accredited. These drugs are given in a number of variations, nonetheless there’s a massive unmet want as sufferers nonetheless have signs regardless of taking these medicines, inflicting disruptions to on a regular basis life. ICS is used to resolve exacerbations regardless of their identified proclivity to trigger pneumonia. LABA/LAMA therapies could trigger cardiovascular and urinary tract dangers.
In keeping with the corporate’s printed analysis, twin inhibition of PDE3 and PDE4 regulates “cAMP and cGMP in airway smooth muscle, which mediates bronchial tone.” Focusing on each of those two collectively has been proven to have higher outcomes than focusing on simply one in every of them, exhibiting enhanced synergistic results.
Financials
VRNA has a market cap of $1.38bn and a cash balance of $257mn. In January, the corporate elevated its present debt facility with Oxford Finance and Hercules Capital to $400mn, $50mn of which it instantly drew. The corporate can additional draw an extra $100M upon approval of ensifentrine, $150M in two separate tranches upon achievement of sure internet gross sales milestones. That, in fact, leaves out one other $100mn, which has not been mentioned. They will even obtain tax credit from the UK tax credit score program, the nation the place they’re primarily based.
Analysis and improvement (“R&D”) bills have been $3.0 million for the third quarter ended September 30, 2023, whereas promoting, basic and administrative bills (“SG&A”) have been $13.4 million. The low R&D bills are attributable to them having accomplished the section 3 trials and never operating any extra trials proper now. Upcoming prices will embrace regulatory and commercialization prices, for which they’re set for funds. At that charge of expense, they’ve a money+debt (funds) runway of 16-18 quarters, which leaves them with ample funds.
Backside Line
An issue I’ve not touched upon right here is that Ensifentrine’s key composition of matter patent expired in 2020, and the corporate plans to carry its personal towards potential ANDA rivals utilizing a patent for the nebulizer formulation, which expires in 2037. Aside from that, knowledge right here appears fairly good and the upcoming PDUFA is a serious catalyst for Verona Pharma plc.